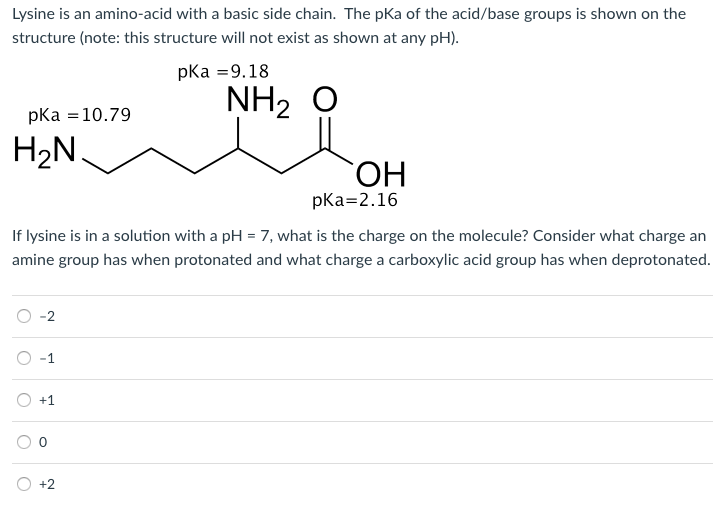
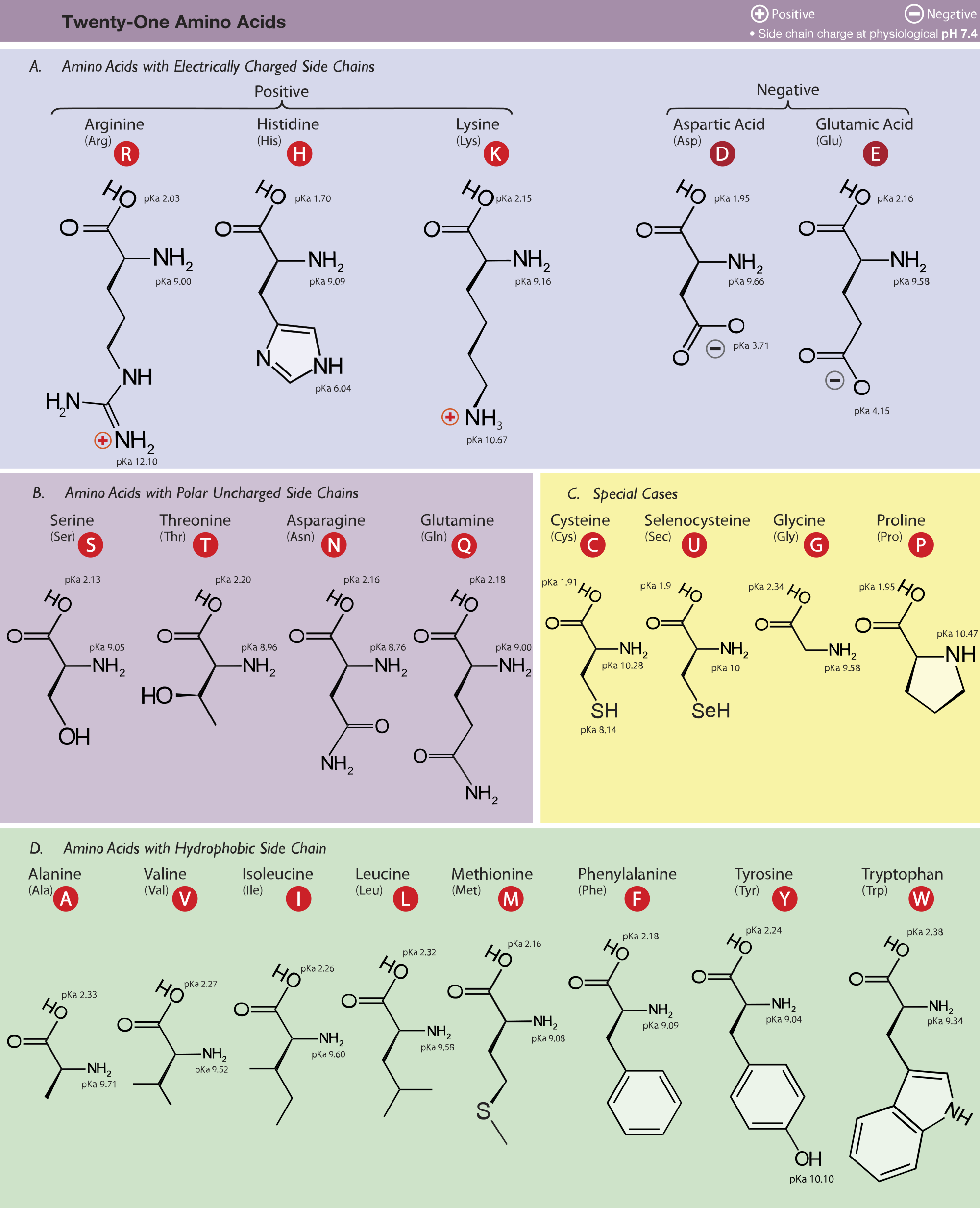
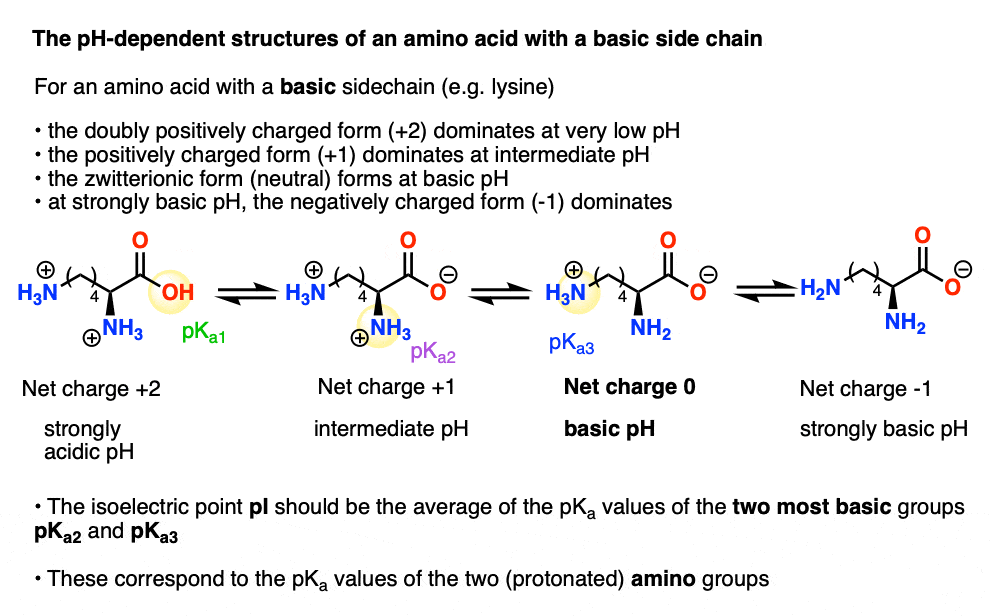
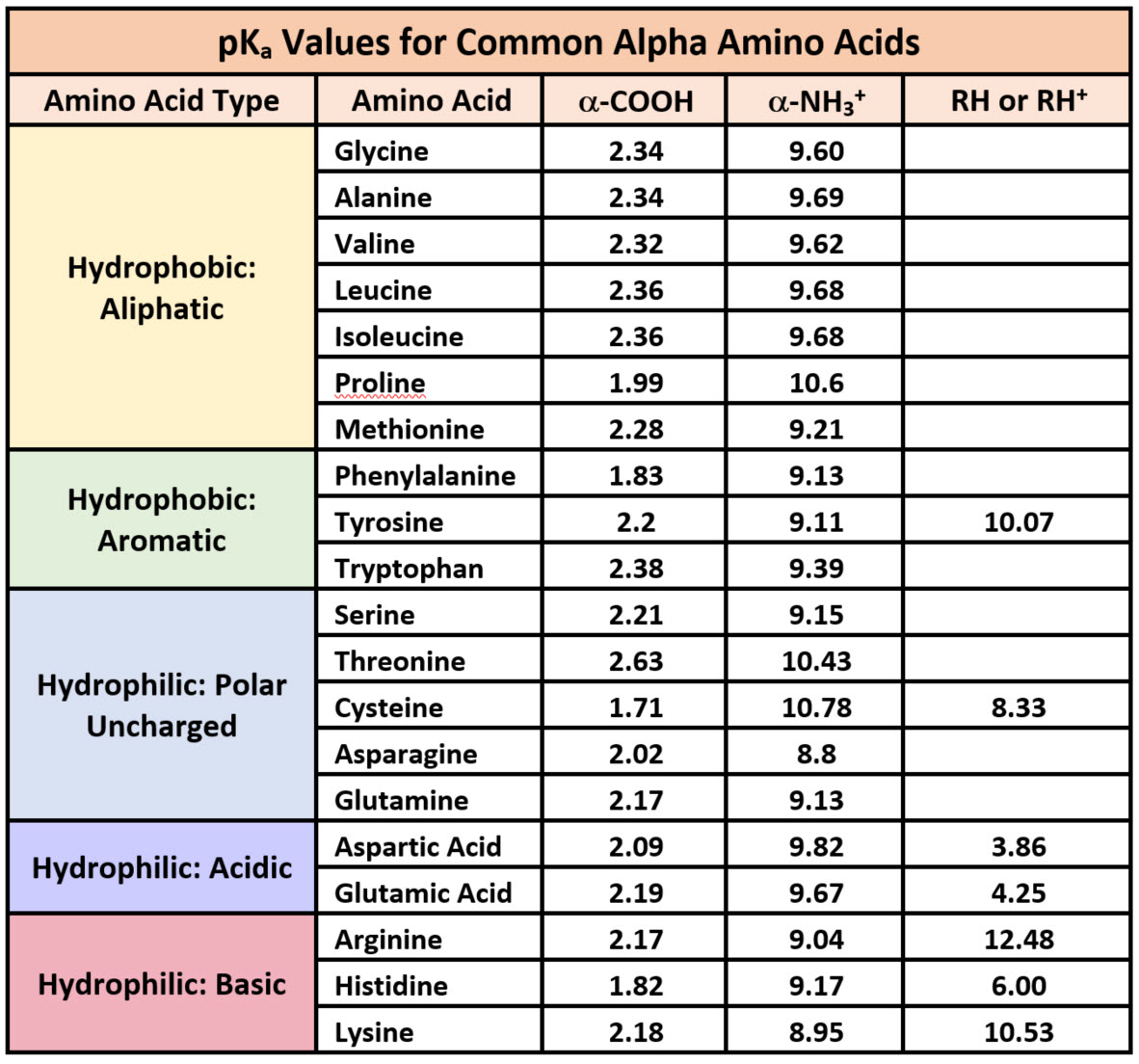
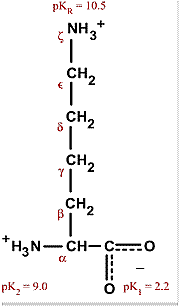
What is the structure of each amino acid at its isoelectric point? (a) alanine (b) methionine (c) aspartic acid (d) lysine | Homework.Study.com

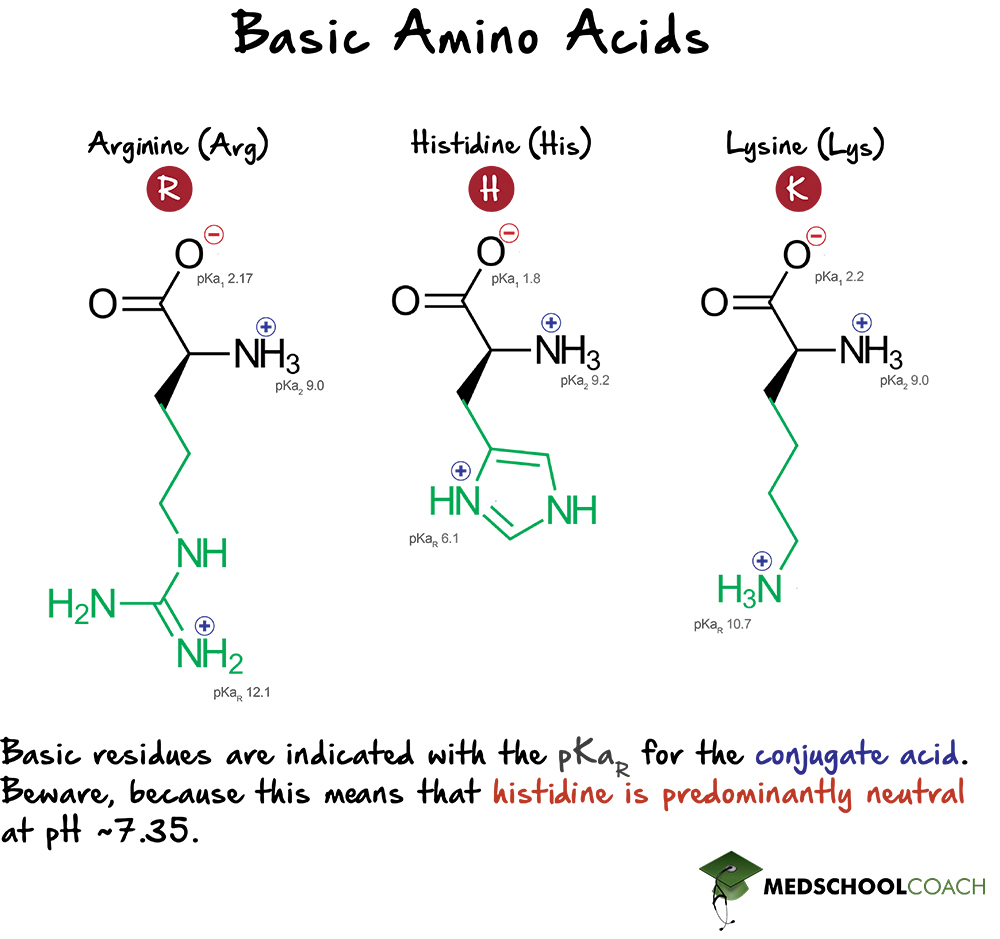
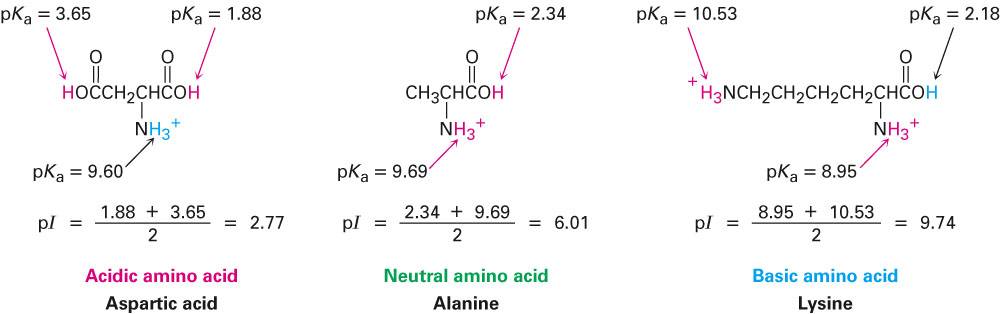
Structure of common basic and acidic amino acids, with the pKa values... | Download Scientific Diagram
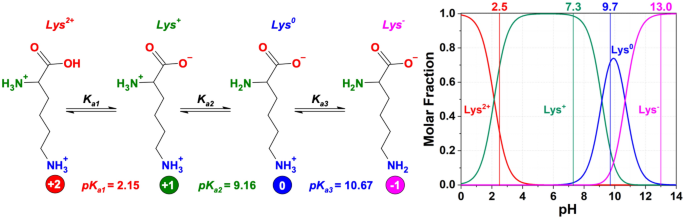
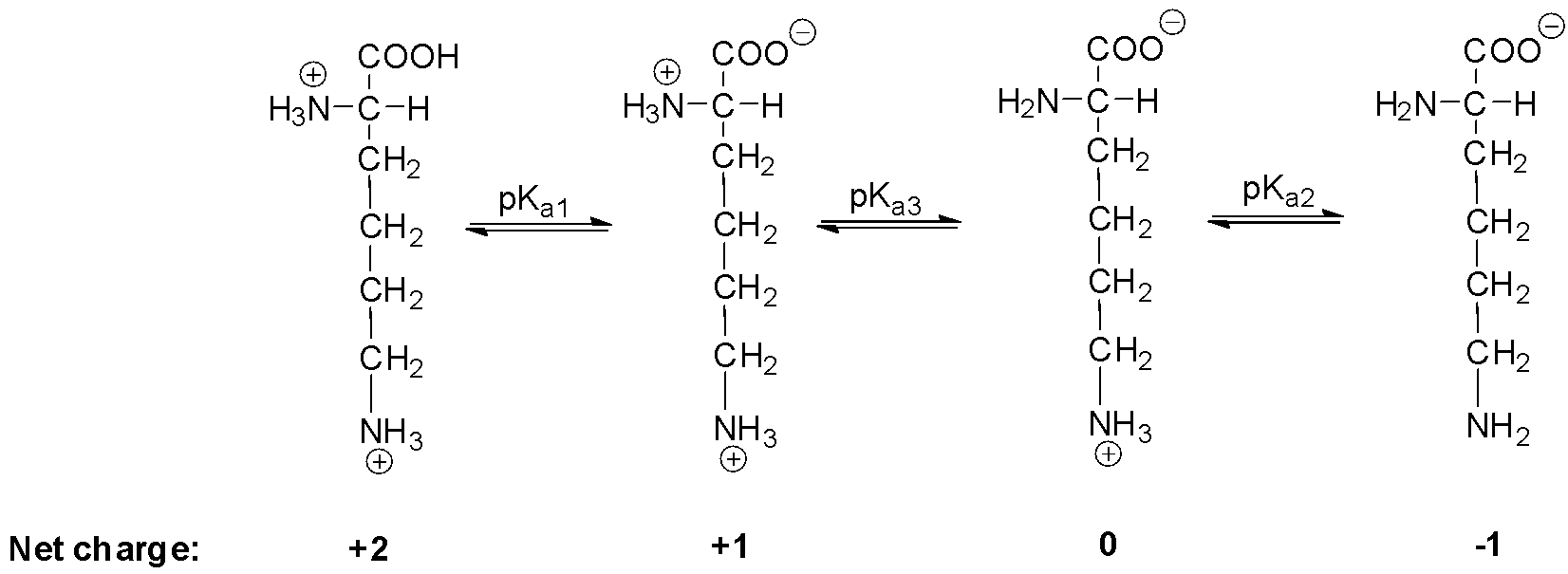
Modulating the poly-l-lysine structure through the control of the protonation–deprotonation state of l-lysine | Scientific Reports